MEDICAL SYSTEMS MANUFACTURING
If you’re into developing products that shape the future of healthcare, welcome to Sanmina Medical. Collaborating with bold thinkers to build products that improve patient outcomes and quality of life is an important part of what we do.
We design, build and service complete medical systems, equipment and products.
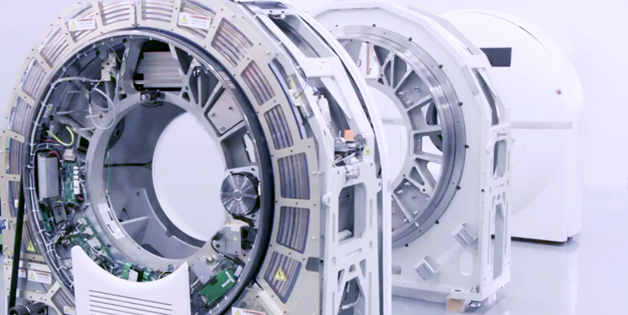
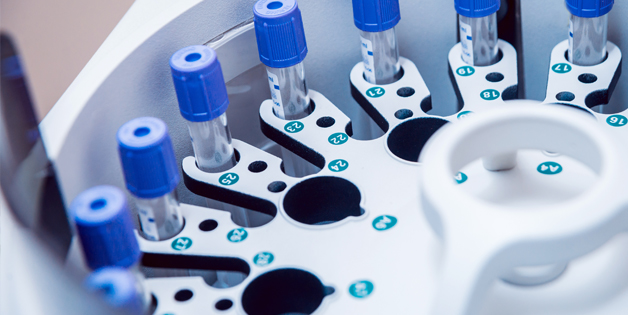
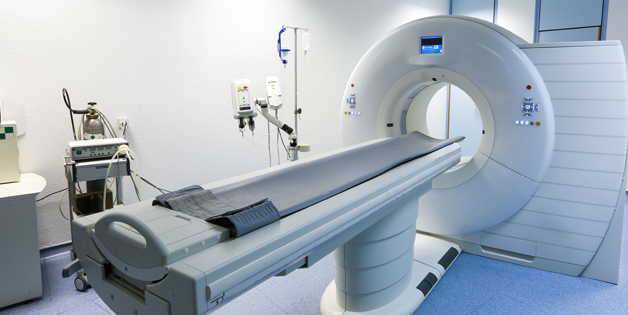
Sanmina produces a broad range of laboratory and point of use blood diagnostics, molecular diagnostics, blood separation and handling equipment. We design and manufacture ultrasound systems, CT, MR, nuclear medicine systems, X-Ray systems, patient monitors and medical devices. For decades Sanmina has been producing personal use and disposable medical devices in high volumes on fully automated and semi-automated production lines.
Global Footprint and Regulatory Compliance
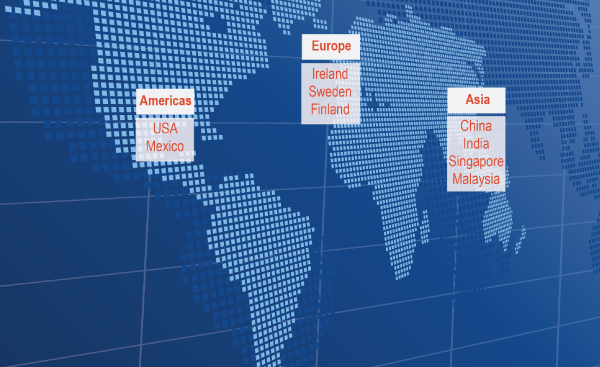
Sanmina operates a global network of ISO 13485 certified medical design and manufacturing facilities. Ten of these facilities are FDA registered. We have medical NPI (new product introduction) and manufacturing facilities in every major region worldwide.
Repair and Refurbishment
Sanmina provides complete repair and refurbishment services for complex medical systems including blood diagnostics systems, CT scanners, X-Ray equipment and many others. We also provide services to manage component obsolescence.
Product Expertise

Blood glucose meters, thermometers and wearable health monitors are manufactured using state of the art fully automated and semi-automated production lines. Sanmina delivers tens of millions of these types of devices every year.
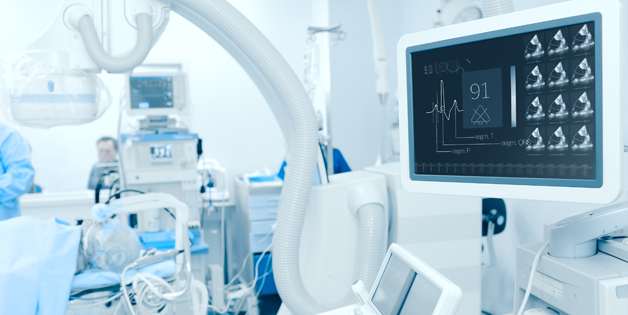
Sanmina has many years of manufacturing experience with hospital, portable and in-home patient monitoring systems. Applications include pulse oxymetry, vital signs monitoring and telemedicine. We have completed multiple board and system designs for various patient monitoring systems.
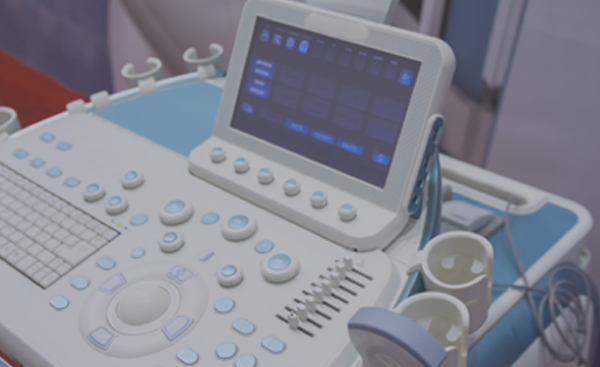
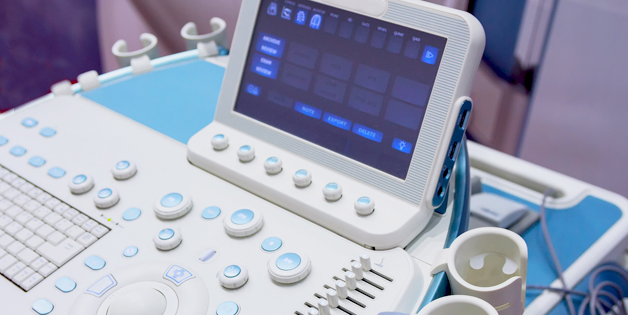
Customers benefit from fully integrated manufacturing services including plastic manufacture, PCB fabrication, PCBA assembly, functional test and system assembly. Sanmina has substantial experience with joint development of ultrasound systems.
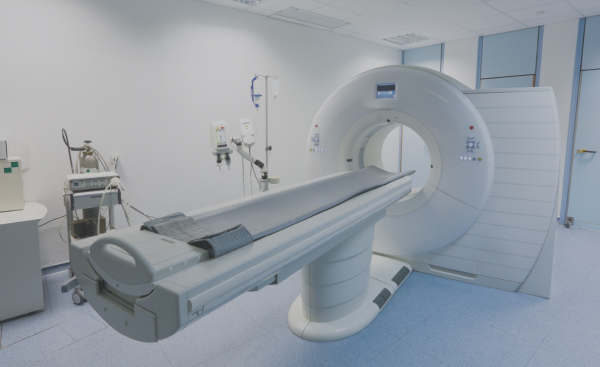
Design, supply chain management, manufacturing, test and repair of large imaging systems including Computed Tomography (CT) scanners, Magnetic, Resonance Imaging (MRI) scanners and X-Ray systems.
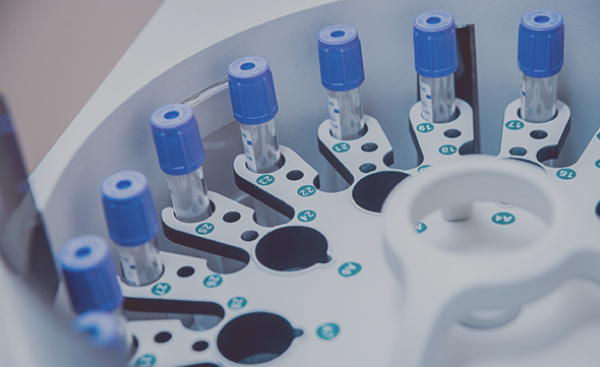
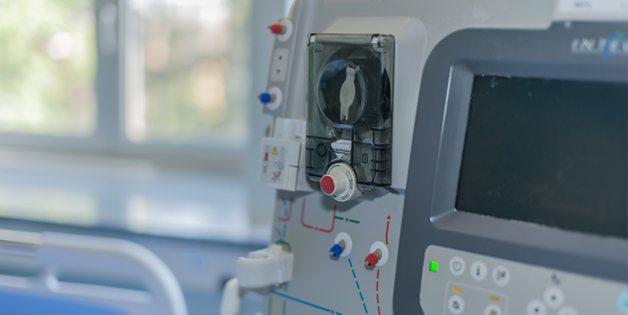
Design, manufacturing & repair of systems with multiple complex technologies including electronics, mechatronics and fluidics. PCB assembly, systems build & test, process development & validation for blood analyzers, immunoassay analyzers and other medical laboratory equipment.
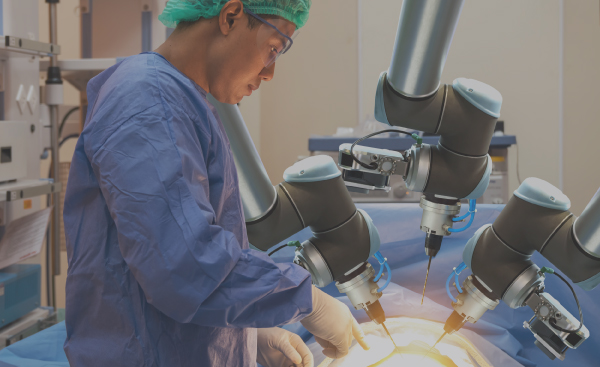
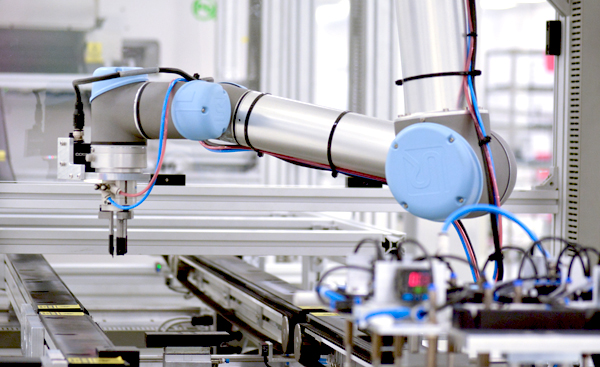
Joint development, systems assembly and functional test of surgical robots and other surgical systems which require the integration of multiple complex technologies including motion control, fluidics, optical systems and electronics.
Quality and Regulatory
Sanmina has implemented a Quality Management System that is ISO 13485 certified and 21 CFR Part 820 and Part 11 Compliant. The QMS utilizes medically trained personnel, standardized metrics, a best in class CAPA system and validated software tools that ensure management throughout a medical product’s lifecycle. The system is maintained and monitored by a dedicated global RA/QA organization.
Manufacturing
Design and Engineering
For many decades, Sanmina has been providing healthcare industry customers with cost-effective, manufacturable designs for medical products. Three of our 12 global design centers are focused solely on medical engineering and are staffed with 160+ dedicated medical engineers.